When delivering nanoparticle drugs to the brain, the blood-brain barrier (BBB) limits the drug transmission very much. Thus, researchers have made great efforts to overcome this issue to improve the efficiency of drug delivery (e.g. the efficiency of nanoparticle drug delivery to cancer cells in tumor tissue through BBB). Recently, a research group from Gachon University have found that there is a difference of permeable capacity by size of gold nanoparticle (AuNP) in the tumorous brain tissue (no difference in the normal brain tissue).
Findings from the new study were published recently in Journal of Drug Targeting through an article titled “Investigation on the effect of nanoparticle size on the blood–brain tumour barrier permeability by in situ perfusion via internal carotid artery in mice”.
As the most important limiting factor in nanoparticle drug delivery to the brain, the BBB is mainly composed of the following cell types: the brain capillary endothelial cells (BCECs), pericytes, astrocytes, and neurons. Among them, BCECs with several specific characteristics contribute the most to the BBB. For example, there are continuous tight junctions between the BCECs, which prevents paracellular transport of substances from the blood to the brain. While in brain tumors, the BBB in the core of a tumor site is more compromised than in the surrounding area and the drug distribution is more restricted than that in other organ tumors. AuNPs are with relatively low cytotoxicity, high water solubility in target cells, easily modified surfaces and tunable light absorption peaks, making them ideal for diagnostic imaging agents or therapeutic agents for experimental genes and drug delivery and photothermal therapy. AuNP-based drug delivery systems have been widely used in the development of cancer chemotherapy due to their high efficacy, low toxicity, high biocompatibility, and customizable designs. In this study, Ji Hee Kang (from Gachon Institute of Pharmaceutical Sciences, Gachon University) investigated the size-dependent AuNP (purchased from CD Bioparticles) permeability across the blood-brain tumor barrier (BBTB) through internal carotid artery (ICA) perfusion in orthotopic-glioblastoma multiforme (GBM) mice by using LSCM for the development of glioblastoma multiforme therapy.
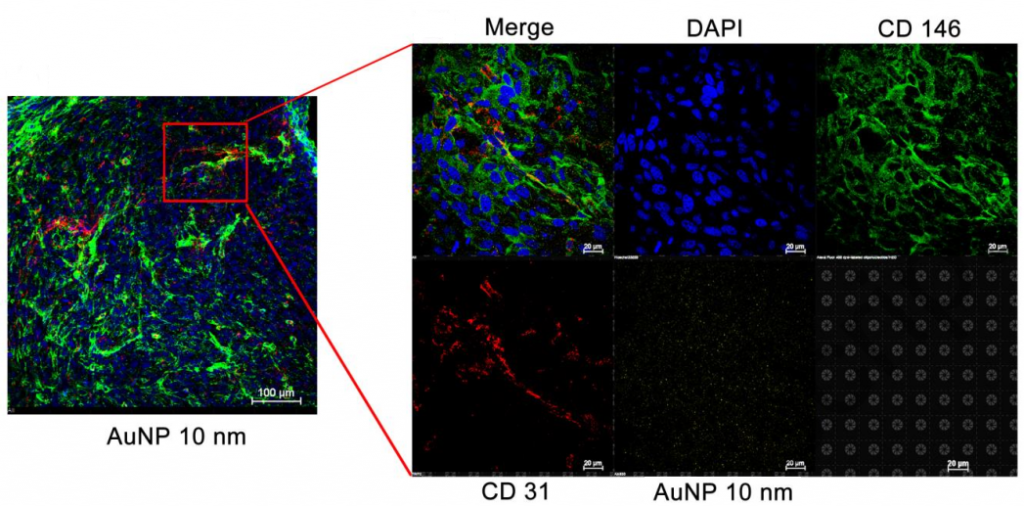
They used a mouse model of GBM, which was established by intracranial implantation of luciferase-expressing human glioblastoma U87MG cells. AuNPs coated with PEG-Alexa 633 were 10, 50, and 100 nm in size and were perfused via ICA of the GBM mice. In normal brain tissue, drugs only permeated across the BBB by passive diffusion while in brain tumors, there was an increasing permeability between microvessels. Then, the distribution of AuNP in these two tissues was observed by confocal microscopy.
They found that “ The permeation of AuNP across the brain endothelial cells is dependent on the size of the nanoparticles. Only 10 nm nanoparticles successfully permeated and spread in the brain tumor, with over 2.3-fold higher number of fluorescence signal that of either 50 or 100 nm nanoparticles” and “The brain section perfused with 10, 50 and 100 nm AuNP showed similar number of blood vessels. All sizes of AuNP were similarly distributed, but the number of AuNP located near the blood vessels was increased with the nanoparticle size suggesting the size-dependent distribution of AuNP in normal brain. The number of AuNP distributed around blood vessels in the normal brain tissue was less than that in the brain tumor tissue. 10 nm AuNP was widely distributed in the brain tissue, whereas 50 and 100 nm AuNPs were aggregately located along the blood vessels.” Thus, small nanoparticles (less than 50 nm) were more efficiently permeated from the blood into the brain, distributing across the brain tumor site.
In conclusion, Kang and his colleagues successfully showed the size-dependent permeability of AuNPs in brain tumor tissue. Moreover, the permeable capacity of AuNPs in the tumorous brain tissue varies by size and no difference showed in the normal brain tissue. Their research presented us with a better understanding of the permeability of nanoparticles across the BBTB and may significantly promote the development of nanoparticle-based drug delivery systems that target brain tumors.
Reference:
Kang, J. H., Cho, J., & Ko, Y. T. (2019). Investigation on the effect of nanoparticle size on the blood–brain tumour barrier permeability by in situ perfusion via internal carotid artery in mice. Journal of drug targeting, 27(1), 103-110.