Gold nanorods (AuNRs) are gold nanoparticles with a specific morphology in the range of 1 to 100 nm. They present two distinct SPRs on the transversal axis and the longitudinal axis. Longitudinal plasmon resonance can provide absorption of wavelength radiation in the near-infrared region (NIR), which is very important in medical applications because the absorption rate of such radiation by living tissue is relatively small. This feature also distinguishes gold nanorods from spherical nanoparticles, making the former suitable for analysis, structural and medical research, and also suitable for metal sol dispersion research. Of course, SPR depends on physical properties such as the size, shape, and thermal stability of nanoparticles. Under the excitation of different laser radiation, the thermal constant decreases significantly, and the particle size ratio increases. The size adjustment can be done by increasing the longitudinal diameter while the absorption spectrum of the NIR region will be enhanced as well. In addition, gold nanorods are very sensitive to the dielectric constant of the surrounding environment, so they can be used as a colorimetric agent.
Gold nanorods are often used in photothermal therapy due to their properties. Among them, combination therapy is considered to be a promising strategy to improve the efficiency of tumor treatment. Therefore, Sim et al. used pH-sensitive polymer, poly(aspartic acid-graft-imidazole)-PEG, to encapsulate gold nanorods and doxorubicin (DOX) to prepare an on-demand pH-sensitive nanocluster (NC) system to improve the efficacy of chemotherapy and photothermal therapy. The results were published in Pharmaceutics. The following are some background introductions and experimental results.
Chemotherapy
Chemotherapy is a drug therapy that uses powerful chemicals to kill fast-growing cells in the body. It is most commonly used to treat cancer because cancer cells grow and multiply much faster than most cells in the body. There are many different chemotherapy drugs to choose from. Chemotherapy drugs can be used alone or in combination to treat a variety of cancers.
Although chemotherapy is an effective way to treat many types of cancer, chemotherapy also carries the risk of side effects. Some chemotherapy has mild side effects (such as nausea, vomiting, diarrhea, hair loss, etc.) and can be treated, while others may cause serious complications (such as lung tissue damage, heart problems, infertility, etc.).
Photothermal therapy
Compared with ultraviolet light and visible light, near-infrared (NIR) light has photons with longer wavelengths and less energy. This feature helps NIR light go deeper into biological tissues, but if there is not enough energy, NIR photons cannot perform many photochemical reactions. Photosensitive nanomaterials can convert NIR light into heat, which can be applied to specific areas for hyperthermia. When nanomaterials are used as nanocarriers in drug delivery systems, the thermally activated nanostructure changes to release the drug. As photothermal agents, the heat produced by these nanomaterials can directly damage or ablate tumor cells. In order to design a qualified PTT system, the photothermal agent needs to meet the conditions of absorption of NIR light, efficient conversion of light and heat, biocompatibility and biodegradability. Examples of these nanomaterials include AuNRs, carbon nanomaterials, and upconversion nanoparticles.
Combination therapies
In recent years, tumor-targeted combination therapy has received more and more attention from researchers, especially in the field of nanomedicine. The combination of multiple therapies is more effective than monotherapy, because it produces a synergistic anti-cancer effect through different mechanisms, reduces drug-related toxicity, and inhibits multidrug resistance. Therefore, using two methods or multiple comprehensive treatment methods or one method to assist another treatment method has become a new trend in cancer treatment. In combination therapy, especially co-chemotherapy and phototherapy, both anticancer drugs and photosensitizers lack tumor selectivity, which increases the potential toxicity to normal tissues. The use of nano-targeted delivery systems to deliver photosensitizers and chemotherapeutic drugs can increase the concentration of drugs at the target, significantly reduce side effects, and improve the effects of phototherapy and chemotherapy.
Their results
In Sim’s research, AuNRs (RFA-10-808, CD Bioparticles) and DOX were encapsulated by a pH-sensitive polymer PAIM-PEG, which improves the efficacy of chemotherapy and photothermal therapy through targeted tumor drug delivery and controlled drug release.
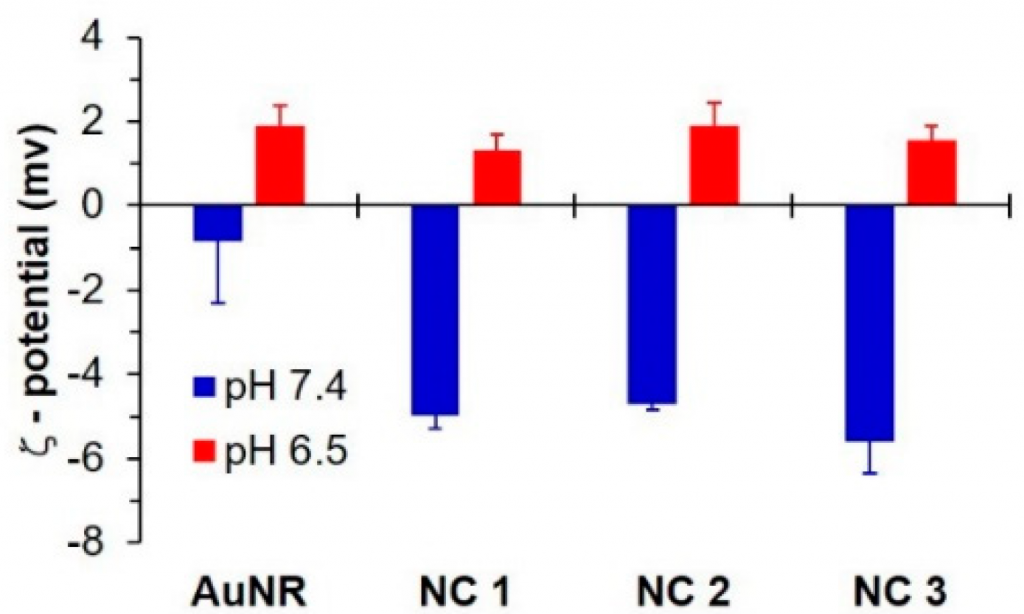
- Zeta potential measurement confirmed the pH sensitivity of the NC systems (Figure 1). At pH7.4, the Zeta potentials of AuNR, NC1, NC2, and NC3 were approximately −0.8, −5.0, −4.7, and −5.6 mV, respectively. When the pH was further reduced to 6.5, the Zeta potentials of AuNR and NC systems increased to about +1.9, +1.3, +1.9, and +1.6 mV, respectively.
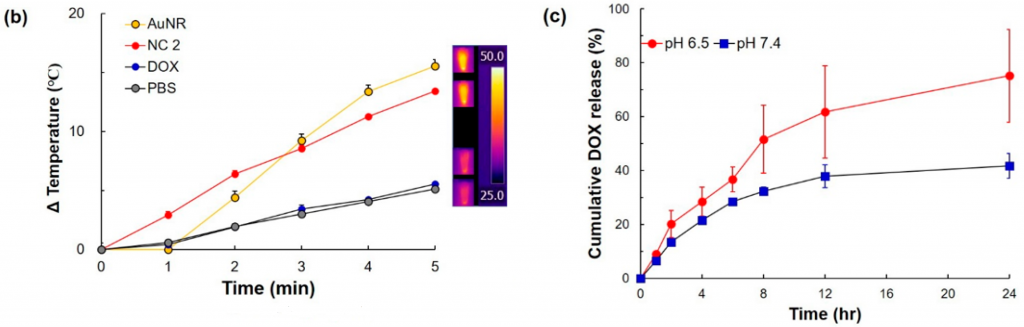
- The NC2, AuNR, and DOX in PBS were irradiated with 808 nm laser with a power density of 2.0 W/cm2 for 5 min, respectively. An infrared camera was used to record the temperature change of the solution (Figure 2b). In order to study drug release, NC2 was exposed to different pH conditions (pH 7.4 and 6.5) (Figure 2c). At pH 7.4, the DOX release profile from the NC system similarly showed less than ca. 40% maximum cumulative release over 24 h, while pH 6.5 caused a significant increase in the DOX release of NC2.
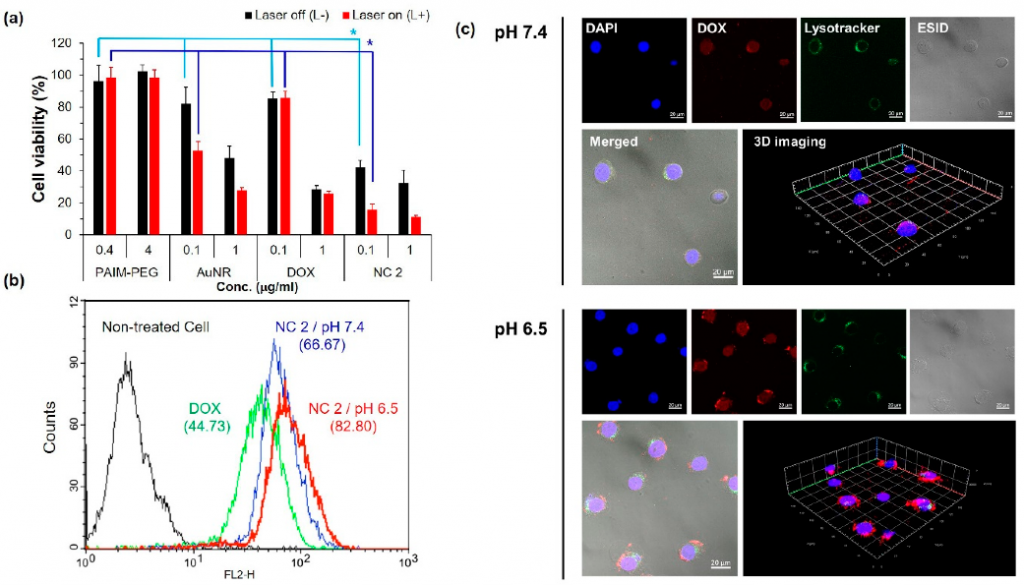
- The cell viabilities of NC2 were evaluated for the synergistic effect of DOX and AuNR. AuNR exhibits the toxicity of the photothermal effect induced by 808 nm laser (Figure 3a), while DOX exhibits similar cytotoxicity regardless of light radiation. Flow cytometry was used to detect the uptake of DOX by live KB cells under different pH conditions to evaluate its synergy. Treating KB cells with NC2 at pH 6.5, the fluorescence intensity was 1.24 times that at pH 7.4 (Figure 3b). LysoTracker®Green DND-26 shows green fluorescence in the endosome and lysosome compartments (Figure 3c).
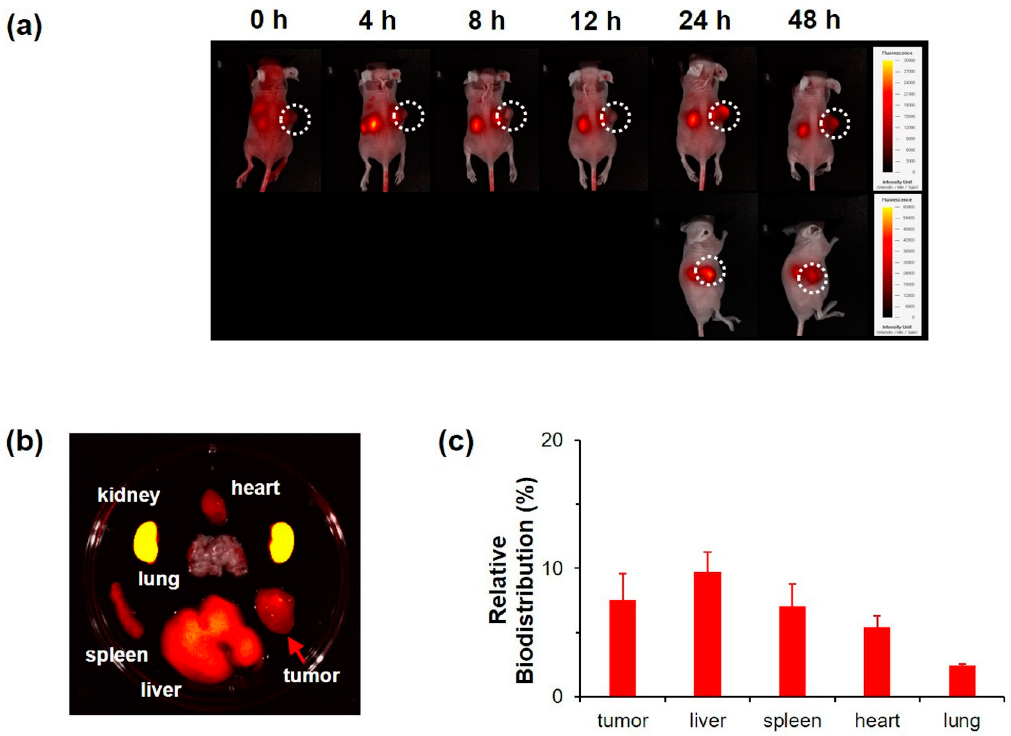
- High-resolution fluorescence imaging was used to study the tumor targeting of NC2 in tumor-bearing nude mice (Figure 4). The results showed that NC2 gradually accumulated in the tumor site after 48h. Representative fluorescence images of various organs showed that NC2 mainly accumulated in the tumor site and the kidney. These results were consistent with the results of in vitro experiments, suggesting that NC2 can inhibit tumor growth in vivo and in vitro.
CD Bioparticles offers a wide range of Functional Gold Nanorods with different active groups including carboxyl, amine, methyl, as well as NHS-activated gold nanorods. These particles are available in different aspect ratios and LSPR absorbance. The carboxyl and amine functional gold nanorods are suitable for covalent conjugation of antibodies, proteins, nucleic acids, and other ligands by using standard EDC/NHS activation. We also provide NHS-activated gold nanorods to enables fast and convenient one-step conjugation of your desired antibody to gold nanorods by a simple mixing process.
Reference:
Sim, T., Lim, C., Hoang, N. H., Shin, Y., Kim, J. C., Park, J. Y., … & Oh, K. T. (2020). An on-demand pH-sensitive nanocluster for cancer treatment by combining photothermal therapy and chemotherapy. Pharmaceutics, 12(9), 839.